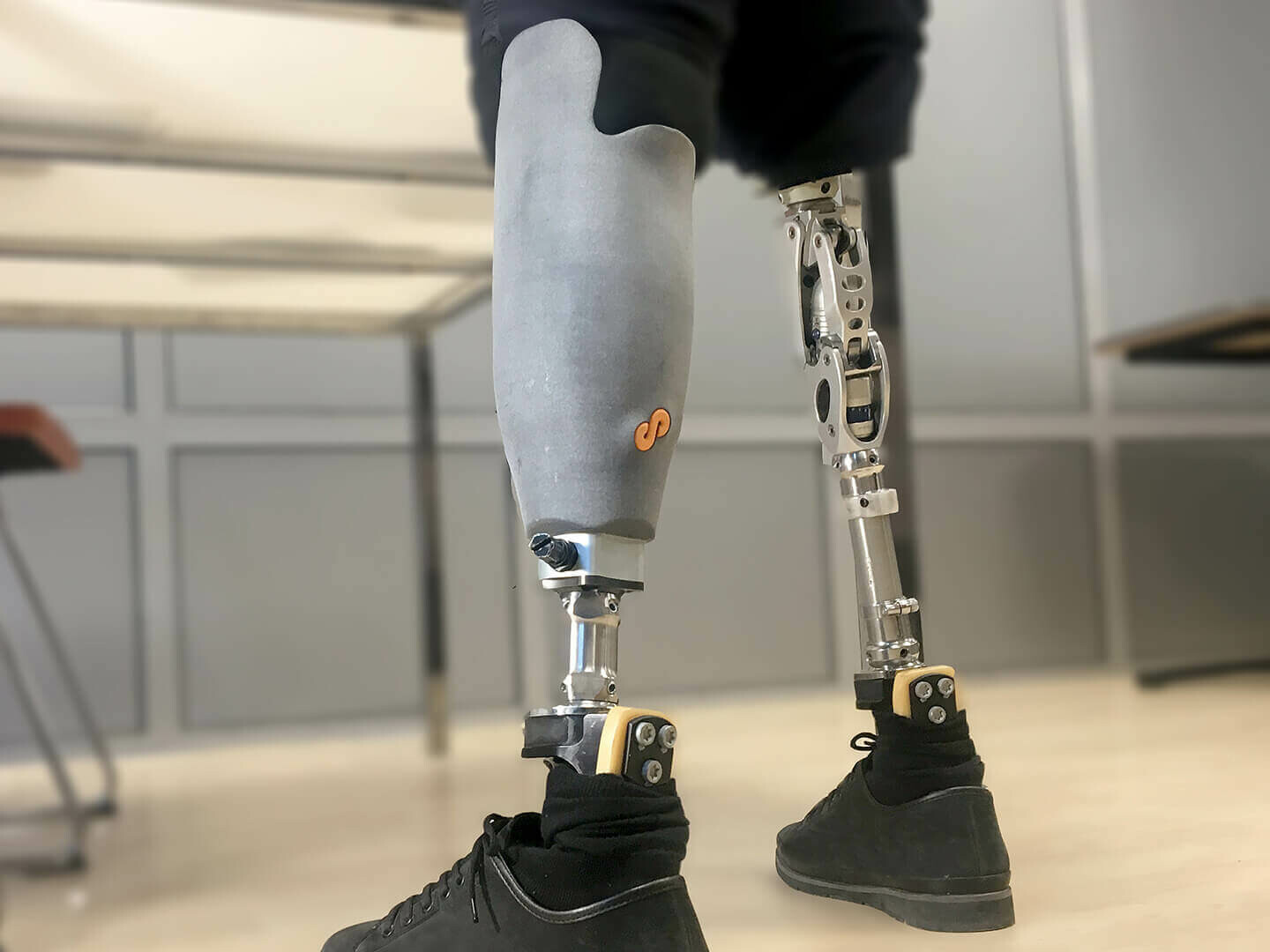
Prosthetics can be made using various materials, including metal alloys, ceramics, plastics, and composites. These materials are chosen based on their strength, durability, and biocompatibility.
Prosthetics have come a long way in recent years, thanks to advancements in materials science and manufacturing technology. Prosthetic limbs, braces, and other devices are now made using durable and lightweight materials that can withstand the stresses of everyday use. The choice of material depends on the patient’s needs, the type of prosthetic, and the part of the body being replaced. For example, metal alloys such as titanium and cobalt-chrome are frequently used for prosthetic joints due to their strength and resistance to wear and tear. Ceramics are often used for dental and cranial implants due to their biocompatibility and durability. Plastics and composites are popular for their flexibility and ease of customization. With so many options available, prosthetists can choose the best materials for their patients and create devices that are both functional and comfortable to wear.
Credit: forward-am.com
Metallic Materials
Metallic materials have long been used in prosthetic devices, owing to their remarkable strength, durability and resistance to wear. As a result, they are used to create various parts of prosthetics, such as joints, bones, and sockets.
Following are some of the metallic materials commonly used to make prosthetic devices:
- Stainless steel: it is one of the most widely used metals in prosthetics due to its corrosion resistance and ability to withstand heavy loads. It is often used in prosthetic joints, screws, and implants.
- Titanium: it is commonly used in prosthetic devices due to its biocompatibility, strength and lightness. It is used in hip replacements, knee replacements, and dental implants.
- Cobalt-chromium: it has a high strength-to-weight ratio and is wear-resistant, making it an excellent option for making prosthetic sockets and discs.
Advantages And Disadvantages Of Metallic Materials
Advantages:
- Durable and long-lasting
- High strength-to-weight ratio
- Biocompatibility
- Offers resistance to wear and corrosion
- Allows for customization and flexibility in design
Disadvantages:
- May cause irritation or allergic reactions in some patients
- Can be expensive
- Can create artifacts on imaging tests, making it difficult to detect bone fractures or problems
- Can cause stress and strain on the patient’s remaining bones and tissues due to the density of the metal
Examples Of Prosthetic Devices Made Of Metallic Materials
Following are some prosthetic devices that are made of metallic materials:
- Hip replacements: these are often made of titanium or cobalt-chromium and can help patients with hip arthritis regain their mobility and reduce pain.
- Dental implants: these are made of titanium and are a popular option for replacing missing teeth.
- Knee replacements: made of a combination of titanium and cobalt-chromium, knee replacements are an effective treatment for knee arthritis.
- Prosthetic sockets: these are made of various metallic materials and are customized to fit the patient’s residual limb, forming the connection between the prosthetic device and the residual limb.
Metallic materials are an excellent choice for designing and manufacturing prosthetic devices. With their strength, durability, and biocompatibility, they can provide a range of benefits for patients with amputations or other limb impairments. However, they also have their limitations and drawbacks, which healthcare professionals and patients should carefully consider before making any decisions.
Polymeric Materials
Polymeric materials are often used in prosthetics due to their excellent elasticity and durability. These materials provide a wide range of benefits that make them a popular choice in the design and production of prosthetic devices. In this post, we will explore the types of polymeric materials used in prosthetics, their advantages and disadvantages, and examples of prosthetic devices that incorporate these materials.
Types Of Polymeric Materials Used In Prosthetics
Polymeric materials used in prosthetics fall into two broad categories: thermoplastics and thermosets.
Thermoplastics
Thermoplastics are polymers that can be melted and re-molded multiple times without incurring any damage to their physical properties.
- Polyethylene
- Polypropylene
- Acrylics
- Polyurethane
- Silicone
Thermosets
Thermosets are polymers that undergo a chemical reaction during manufacture which cross-links the polymer chains, making them stable and un-melted.
- Epoxy resins
- Polyester resins
- Phenolic resins
Advantages And Disadvantages Of Polymeric Materials
Polymeric materials offer several benefits in prosthetics.
- Efficient and cost-effective production process
- Durable and long-lasting
- Lightweight and easy to manipulate.
- High biocompatibility
- Excellent wearing comfort
- Improved aesthetic appeal
However, there are also some limitations to the use of polymeric materials in prosthetics.
- Limited strength and stiffness
- Less resistance to heat and breakage
- More susceptible to wear and tear over extended usage
Examples Of Prosthetic Devices Made Of Polymeric Materials
There are many types of prosthetic devices made of polymeric materials.
- Silicone-based prosthetic liners for amputees
- Epoxy-laminated prosthetic sockets
- Polyethylene-based prosthetic feet
- Polyester-supported prosthetic hands
- Polyurethane-based prosthetic knees
In Conclusion
Prosthetics have come a long way, and polymeric materials are at the forefront of prosthetic innovation. The types of polymeric materials used in prosthetics, their advantages and disadvantages, and examples of prosthetic devices made of these materials are critical to understanding the role of polymers in prosthetics.
From providing improved mobility to improving the wearer’s quality of life, polymeric materials have come a long way in reshaping the lives of amputees.
The Basics of a Below-knee Prosthetic Leg
Carbon Fiber Reinforced Polymer (Cfrp) Materials
What Is Cfrp And Why It Is Used In Prosthetics
Carbon fiber reinforced polymer (cfrp) is a material made up of carbon fibers and polymers. It is widely used in industries that require lightweight yet durable materials such as in aerospace, automobile, and even medical devices. Cfrp is an excellent material for prosthetic devices because of its unique physical properties.
- Cfrp offers a high strength-to-weight ratio, making it a lightweight yet sturdy material for prosthetic devices.
- It can withstand heavy loads, reducing the risk of breakages or damages.
- The material is corrosion-resistant, allowing it to last longer.
Benefits Of Cfrp For Prosthetic Devices
Cfrp has become a popular material for prosthetics because of its many benefits that can improve an amputee’s quality of life.
- Lightweight: cfrp is extremely light, making it ideal for prosthetics. It allows for a comfortable fit and eases the burden on the patient’s remaining limb.
- Durability: it is a strong and long-lasting material that can hold up against continuous use and wear.
- Versatility: cfrp can be molded into any shape, making it easy to customize and tailor to each patient’s unique needs.
- Better mobility: the use of cfrp in prosthetics allows for better mobility, making it easier for the patient to walk, run, and engage in other activities without added pain or discomfort.
Examples Of Prosthetic Devices Made Of Cfrp Materials
The following are some of the most common prosthetic devices that utilize cfrp material:
- Prosthetic legs: most prosthetic legs include cfrp material. These materials can be extended or shortened depending on the amputee’s needs, providing them with comfortable flexibility.
- Artificial hands: cfrp is also used in the development of artificial hands. The material is lightweight and robust, making it easier for patients to carry out daily activities.
- Back braces: cfrp is used in the production of spinal braces. These braces can be customized to support different parts of the back, applying much less pressure than traditional spinal braces.
Cfrp is a versatile material that has proven useful in many different industries. Its use in prosthetic devices has improved amputees’ lives by providing them with lightweight, durable prosthetics that allow them to move freely and comfortably.
Silicone
What Is Silicone And Why It Is Used In Prosthetics
Silicone is a material that is commonly used in prosthetics due to its unique properties. It is a synthetic polymer made from silicon, oxygen, and other elements like carbon and hydrogen. Is flexible, durable, and can be easily molded into different shapes and sizes.
The use of silicone in prosthetics is highly favored because of its biocompatibility, meaning it does not react with the human body, it is hypoallergenic and has excellent color-matching capabilities, which make it the perfect material to create lifelike skin for prosthetic devices.
Advantages And Disadvantages Of Silicone Materials
Advantages:
- Excellent color matching capabilities
- Flexible and durable
- Hypoallergenic and biocompatible
- Water-resistant
- Can be easily molded into different shapes and sizes
Disadvantages:
- Can be expensive compared to other materials
- The heat retention of silicone can cause sweating and discomfort in hot weather conditions
- Requires specialized equipment and skilled professionals to create the prosthetic device
Examples Of Prosthetic Devices Made Of Silicone
Silicone is commonly used in prosthetic devices due to its versatility and the benefits it offers.
- Silicone breast prosthesis
- Silicone facial prostheses such as ears, noses, and eyes
- Silicone finger and toe prostheses
- Silicone liners used in prosthetic limbs
- Silicone facial masks used for patients with burn injuries or undergoing post-surgical recovery.
Silicone has made significant advancements in the prosthetic industry, providing patients with comfort, function, and aesthetics.
Composites
Prosthetic devices have become a life-changing solution for people with disabilities, allowing them to perform daily activities with greater ease and independence. As technology continues to advance, so too does the range of materials used to create prosthetic devices. Composite materials, in particular, offer a unique set of advantages and disadvantages worth exploring.
In this section, we’ll take a closer look at the types of composite materials used in prosthetics, their advantages and disadvantages, and some examples of prosthetic devices made from composites.
Types Of Composite Materials Used In Prosthetics
Composite materials are made up of two or more substances with different physical or chemical properties, combined to create a new material with unique characteristics.
- Carbon fiber reinforced polymers (cfrp): combining carbon fiber and plastic or resin results in a material that is lightweight, strong, and durable, making it ideal for creating prosthetic limb sockets, orthotics, and other supportive elements.
- Fiberglass reinforced composites (frc): a combination of fiberglass fibers and resin is used to create a flexible, lightweight, and strong material that can be shaped to match specific body parts, making it suitable for creating prosthetic devices that require a custom fit, such as limb liners and braces.
Advantages And Disadvantages Of Composite Materials
While composite materials offer several advantages, they also have some drawbacks worth noting.
Advantages:
- Lightweight: composite materials are lighter than many traditional materials, making them more comfortable for the wearer and requiring less energy to move.
- Durable: composite materials are strong and can withstand wear and tear, reducing the need for frequent replacement.
- Customizable: composite materials can be molded to fit specific shapes, making them ideal for creating prosthetic devices with a custom fit.
- Corrosion-resistant: composite materials are less prone to corrosion than metals, reducing the risk of device failure.
Disadvantages:
- Costly: composite materials can be expensive, making them less accessible to some who need them.
- Difficult to repair: the unique properties of composite materials can make them difficult to repair if damaged.
- Limited recycling options: unlike metals, composite materials are not easily recyclable, contributing to waste and environmental concerns.
Examples Of Prosthetic Devices Made Of Composite Materials
Here are some examples of prosthetic devices made of composite materials:
- The össur pro-flex xc torsion foot: this prosthetic foot is made of a carbon fiber composite and offers flexibility and energy return for improved walking comfort.
- The college park odyssey k2: this prosthetic foot is made of a fiberglass composite, offering a durable and lightweight solution for active individuals.
- The freedom innovations plie iii: this prosthetic knee joint is made of a carbon fiber composite and offers enhanced stability and mobility for users.
Composite materials offer a unique set of advantages and disadvantages when it comes to their use in prosthetics. They are lightweight, durable, and customizable, but can also be costly and difficult to repair. Nevertheless, composite materials continue to be a popular choice for creating prosthetic devices that meet the needs of individuals with physical disabilities.
Acrylic Resins
Acrylic resins are widely used in prosthetics manufacturing worldwide. They are a popular choice for the production of prosthetic devices such as dentures, implant overdentures, artificial eyes, and facial prosthetics. Acrylic resins are an advantageous material used for prosthetics due to their affordability, durability, ease of use, and versatility.
What Are Acrylic Resins And Why They Are Used In Prosthetics
Acrylic resins are a type of plastic that is created when a liquid monomer is added to a polymer powder. This mixture polymerizes into the solid acrylic resin with the help of heat or ultraviolet light. These resins can be easily molded into different shapes and sizes that are required in prosthetic devices production.
- They can be easily shaped to any size or form.
- They are lightweight yet strong.
- They are durable and can withstand daily wear and tear.
- They are non-toxic and hypoallergenic.
- They are easy to manipulate and repair if they get damaged.
- They have a natural-looking appearance that blends well with surrounding tissues.
Advantages And Disadvantages Of Acrylic Resins
Like all prosthetic materials, acrylic resins have advantages and disadvantages.
Advantages
- Acrylic resins are a cost-effective and affordable option for prosthetic creation.
- They are strong and durable, which ensures the longevity of prosthetic devices.
- They can be easily adjusted or modified without requiring a lot of time and effort.
- They are available in a range of colors and shades to match the natural appearance of surrounding tissues.
Disadvantages
- They are susceptible to staining from food or drink and may require frequent polishing to maintain their appearance.
- They can be brittle and prone to cracking or breaking if subjected to excess pressure.
- They may not be suitable for patients with sensitive skin, as they can cause allergic reactions.
Examples Of Prosthetic Devices Made Of Acrylic Resins
Acrylic resins are used in the production of various prosthetic devices.
- Dentures: acrylic resin is the most commonly used material in the production of dentures.
- Implant overdentures: acrylic resin is used to secure full or partial dentures on dental implants.
- Facial prosthetics: acrylic resin is used to create artificial ears, noses, and eye prostheses.
- Orthotics: acrylic resin is used in the production of splints, braces, and other supportive orthotic devices.
Acrylic resins are a suitable material for prosthetic devices that require strength, versatility, and a natural appearance. With their range of advantages and few disadvantages, they are a popular choice in creating prosthetic devices for various conditions.
Thermoplastic Elastomers (Tpes)
Thermoplastic elastomers (tpes) are a specific type of material used in prosthetics. They are a combination of plastic and rubber materials that have unique properties that make them suitable for prosthetic devices. Let’s take a closer look at what tpes are and why they are commonly used in prosthetics.
What Are Tpes And Why They Are Used In Prosthetics
- Tpes stand for thermoplastic elastomers, which are a blend of plastic and rubber materials.
- They are used in prosthetics because of their flexibility, durability, and biocompatibility properties.
- Tpes have the ability to stretch and return to their original form without breaking or losing shape, making them ideal for prosthetic devices that require both rigidity and flexibility.
- They are also known for their resistance to chemicals, uv radiation, and extreme temperatures, making them ideal for outdoor prosthetic use.
Advantages And Disadvantages Of Tpes
Advantages:
- Tpes are non-toxic and biocompatible, meaning they can be safely worn by prosthetic users and do not harm the body.
- They are highly customizable and can be molded into a variety of shapes and sizes.
- Tpes are flexible and can be stretched without breaking, making them ideal for prosthetic use.
- They are easily processed, making them cost-effective.
Disadvantages:
- Tpes are not as strong as other materials used in prosthetics, such as carbon fiber or metal alloys.
- They can wear out faster than other materials, especially with heavy use.
- Tpes have a lower heat resistance compared to other materials, which may cause them to deform when exposed to high temperatures.
Examples Of Prosthetic Devices Made Of Tpes
- Liners: tpe liners are used in socket liners to provide cushioning and reduce pressure. They are highly flexible and conform to the shape of the residual limb, reducing discomfort during use.
- Covers: prosthetic covers made of tpes improve the aesthetic appearance of a prosthetic limb. They can be customized to match skin tone and are highly durable.
- Joints: tpe joints are used in prosthetic limbs to provide flexibility and range of motion. They are commonly used in knee and ankle joints.
- Grips: tpe grips are used on the underside of prosthetic limbs to provide traction and prevent slipping.
Prosthetic devices made of thermoplastic elastomers (tpes) have advantages and disadvantages, but they are a popular choice in the prosthetic industry. Tpes are highly customizable, biocompatible, and flexible, making them perfect for prosthetic covers, liners, joints, and grips.
Natural Materials
Natural materials have been used since the beginning of prosthetics to create sturdy, flexible, and cost-effective prosthetic limbs for people with disabilities. Natural materials in prosthetics add a touch of familiarity and authenticity that can’t be matched by synthetic options.
Here are some of the types of natural materials used in prosthetics.
Types Of Natural Materials Used In Prosthetics
- Wood: wood remains a popular and inexpensive natural material used in prosthetics manufacturing since ancient times. The manufacturing process involves carving wood into the desired shape before attaching it to other prosthetic parts.
- Rubber: natural rubber is commonly used in prosthetics due to its malleability and durability. The material’s resilience makes it an excellent cushioning material around joints.
- Leather: leather is often used in prosthetic coverings rather than the prosthetic device itself. Leather creates a soft, comfortable feel against the skin and is known for its wear-resistance.
- Linen: linen is a stiff, strong, and lightweight material used in prosthetics manufacturing. It has been used for centuries in the creation of prosthetic arms, given its capacity for holding its shape.
- Carbon fiber: carbon fiber is increasingly gaining popularity as a natural material in prosthetic devices. It is strong yet lightweight and can be molded into any shape, making it ideal for prosthetic parts such as feet and hands. Carbon fiber also has excellent shock absorbency, which is crucial in prosthetic legs.
Advantages And Disadvantages Of Natural Materials
Advantages:
- Natural materials are often inexpensive in comparison to other materials like carbon fibers or metals.
- Natural materials can provide a comfortable and cushioning feel on contact with the skin, ideal for amputees with sensitive skin.
- Natural materials can also offer excellent shock absorbency.
Disadvantages:
- Natural materials are prone to wear and tear, thus requiring frequent replacements that can be costly.
- Natural materials may not be long-lasting or durable in adverse weather conditions or high-stress applications.
- Natural materials may also be less adjustable, making comfortability and flexibility an issue for the user.
Examples Of Prosthetic Devices Made Of Natural Materials
- Hosmer arm: the hosmer arm is one of the most popular prosthetic arms made with wood. It is known for its flexible shoulder joints, strong elbow joints, and stability in its grippers.
- Jaipur foot: jaipur foot takes inspiration from human anatomy and is made with rubber, wood powder, and various plastics. It is cost-effective and offers excellent shock absorbency and flexibility for the user.
- Transmetatarsal prostheses: transmetatarsal prostheses are made with carbon fiber and leather. They offer comfortability, shock absorbency, and durability, making them perfect for amputees with flexible ankles and foot prosthetics.
Natural materials in prosthetics have come a long way, with many advancements in manufacturing techniques and materials handling processes. Despite their limitations, natural materials remain a viable option for prosthetic solutions, especially concerning cost-effectiveness and comfortability.
Advanced Materials
Overview Of Advanced Materials Used In Prosthetics
Prosthetic devices have come a long way in recent years, thanks in part to a range of advanced materials used in their construction. These materials are often lightweight, durable, and flexible, making them ideal for use in prosthetics.
- Advanced materials used in prosthetics are often engineered or synthetic, rather than natural.
- Materials such as titanium, carbon fiber, and silicone are commonly used in prosthetics.
- These materials can be manipulated to create a wide range of prosthetic devices, from arms and hands to legs and feet.
Benefits And Challenges Of Using Advanced Materials
As with any technology, there are both benefits and challenges to using advanced materials in prosthetics.
- Benefits:
- Advanced materials are often more durable and lightweight than traditional prosthetic materials.
- These materials can be manipulated to create prosthetic devices that are tailored to the specific needs of the user.
- They can often be manufactured using 3d printing technology, which allows for quick and relatively low-cost production.
- Challenges:
- Advanced materials can be more expensive than traditional prosthetic materials.
- Some advanced materials may require specialized training or equipment for production.
- The production of advanced prosthetic devices can sometimes be complex, requiring careful attention to detail to ensure that the device functions as intended.
Examples Of Prosthetic Devices Made Of Advanced Materials
There are many examples of prosthetic devices that are made using advanced materials.
- The i-limb hand, made by touch bionics, is a prosthetic hand that uses a range of advanced materials, including silicone and carbon fiber, to create a hand that is both lightweight and strong.
- The c-leg, made by otto bock, is a prosthetic leg that uses a microprocessor to control the movement of the knee joint. The leg is made using lightweight materials, including carbon fiber, to minimize the weight of the device.
- The biom t2 system, made by össur, is a prosthetic ankle-foot system that uses advanced materials and technology to mimic the movement of the human ankle. The device is made using a range of materials, including titanium, carbon fiber, and silicone.
Advanced materials play a vital role in the development of modern prosthetic devices. By providing a range of benefits, including increased durability and flexibility, these materials are helping to make prosthetic devices more accessible and effective than ever before.
Future Of Materials In Prosthetics
Advancements in materials have revolutionized the field of prosthetics. The use of materials like carbon fiber, titanium and silicone have allowed the creation of advanced prosthetic devices that are lighter, stronger and more comfortable for the user. But what does the future hold for materials in prosthetics?
Overview Of The Latest Advancements In Materials Used For Prosthetics
Here are some of the latest advancements in prosthetic materials:
- 3d-printing: 3d-printing has the potential to create custom prosthetic devices that are perfectly fitted to the user’s limb. This technology is already being used in the development of advanced prosthetic sockets.
- smart materials: smart materials can change their properties in response to external stimuli like temperature, light or electric fields. These materials can be applied in prosthetics to allow the device to self-adjust, reducing the need for manual adjustments.
- graphene: graphene is a form of carbon that is incredibly strong, flexible and electrically conductive. Researchers are exploring the use of graphene in prosthetics due to its potential to create lightweight yet strong prosthetic devices.
Potential Of Materials Science For Advancing Prosthetic Devices
Materials science has the potential to revolutionize the field of prosthetics by unlocking new possibilities for prosthetic devices like:
- bionic limbs: bionic limbs are robotic prosthetic devices that could replace the functions of lost limbs. With advancements in materials science and robotics, it is possible to create bionic limbs that function almost as well as a natural limb.
- self-powered prosthetics: materials that can generate power from movement, such as piezoelectric materials, have the potential to create self-powered prosthetic devices that do not require an external power source.
- regenerative prosthetics: materials that support the growth of human tissue, such as biodegradable scaffolds, have the potential to create regenerative prosthetic devices that can heal the user’s tissues over time.
The future of materials in prosthetics is exciting, with new advancements and possibilities emerging regularly. With continued advancements in materials science, prosthetic devices are set to become more advanced, efficient, and comfortable than ever before.
Frequently Asked Questions Of Types Of Materials Used In Prosthetics
What Are Some Common Materials Used In Prosthetics?
Prosthetics are made from various materials such as silicone, carbon fiber, titanium, and plastics. These materials offer different benefits and can be customized to meet the needs of individuals. The choice of material depends on the type of prosthetic required and the level of activity it will be used for.
How Do The Materials Used In Prosthetics Affect Their Durability And Lifespan?
The materials used in prosthetics affect their durability and lifespan greatly. Materials like titanium and carbon fiber are durable, lightweight, and long-lasting. However, materials like plastic and rubber are less durable and may need frequent replacements. Careful consideration must be given to the choice of materials to ensure the longevity of the prosthetic.
Are There Any Materials Used In Prosthetics That May Cause An Allergic Reaction?
Yes, some materials used in prosthetics such as nickel, titanium, and latex may cause an allergic reaction. It is important to inform a prosthetist about any allergies so they can choose suitable materials for the prosthetic device. Regular monitoring is also necessary to ensure any discomforts are addressed.
Can Prosthetic Materials Be Customized To Fit Individual Patient Needs?
Yes, prosthetic materials can be customized to fit individual patient needs, based on factors such as size, shape, and lifestyle requirements. Customization ensures comfort and optimal functionality for the user and can be achieved through various methods, such as 3d printing technology and prosthetist expertise.
How Do Advances In Materials Science Affect The Development Of New Prosthetics?
Advances in materials science have revolutionized the development of new prosthetics. With improved materials such as carbon fiber, titanium and silicon, prosthetics can now be created to mimic natural movements and sensations of the limbs. This has greatly enhanced the quality of life for amputees.
Are There Any Environmental Concerns Associated With The Disposal Of Prosthetic Materials?
Yes, prosthetic materials can pose environmental concerns if not disposed of properly. Some prosthetics contain metals and plastics that can release harmful chemicals when decomposing. Recycling and proper disposal methods can help mitigate these concerns.
What Are Some Of The Challenges Facing Prosthetic Designers And Engineers In Terms Of Materials Selection?
Prosthetic designers and engineers face challenges in selecting materials that meet the physical demands of the user’s activities. Materials must also be biocompatible, lightweight, durable, and cost-effective. Balancing these requirements to create a prosthetic that is functional, comfortable, and aesthetically pleasing presents a significant challenge.
How Do The Materials Used In Prosthetics Affect The Cost Of The Device?
The materials used in prosthetics can significantly affect the cost of the device. High-quality materials such as carbon fiber or titanium increase the cost, while lower-quality materials like plastic or aluminum reduce it. The durability, weight, and functionality of the prosthetic also depend on the materials used, which can affect the overall cost.
Conclusion
As technology advances, the materials used in prosthetics continue to evolve and improve. While there are a variety of materials used in the construction of prosthetic limbs and devices, each has its own unique advantages and disadvantages. When choosing a material, the degree of comfort, weight, durability, and overall effectiveness will all need to be considered.
The most common materials used in prosthetics include carbon fiber, titanium, silicone, and polyethylene. When choosing which material is best suited to the needs of an individual, factors such as their activity level, lifestyle, and budget will all play a role.
It is important to consult with a healthcare professional to determine the best material for each specific situation. With the use of advanced materials, prosthetic devices can greatly improve the quality of life for those in need of them.