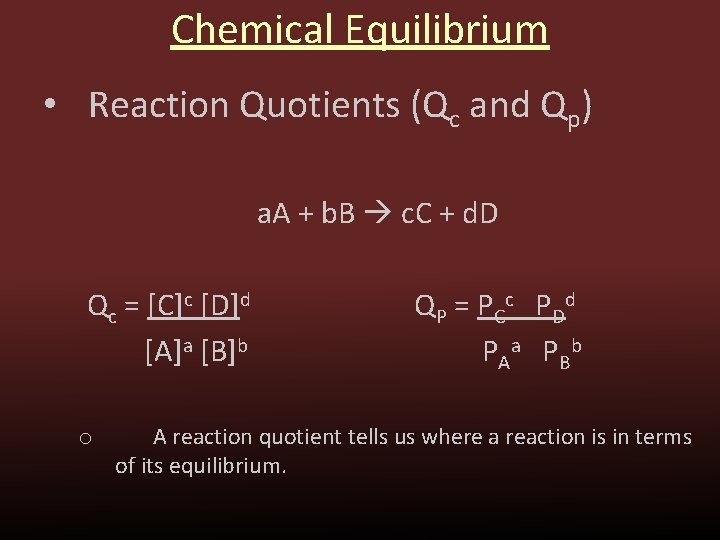
In chemistry, Qp is the symbol for heat capacity at a constant pressure. Heat capacity is the amount of heat needed to raise the temperature of a substance by one degree. The pressure-volume product (PV) is a measure of how much work a system can do.
Work is done when a force moves an object through a distance, and it is equal to the force times the distance. In order to calculate Qp, you will need to know the value of PV and the change in temperature (ΔT).
- There are a few steps in calculating Qp in Chemistry
- They are as follows: 1) Firstly, calculate the change in enthalpy (ΔH)
- This can be done by using the equation ΔH = qp + w
- 2) Next, calculate the change in entropy (ΔS)
- This can be done by using the equation ΔS = -q/T
- 3) Finally, calculate Qp by using the equation Qp = ΔH – TΔS
Credit: slidetodoc.com
What is Qp in Chem?
In chemistry, QP is short for quality control. Quality control is a process by which a company or organization ensures that its products meet certain standards of quality. This can be done through testing, inspection, and/or feedback from customers.
Quality control is important in many industries, but especially in those where safety is a concern (e.g., food and pharmaceuticals).
QP chemists are responsible for ensuring that the products their companies produce meet all relevant quality standards. This can involve testing the products to ensure they meet specifications, conducting research to develop new and improved methods of quality control, and investigating customer complaints.
In some cases, QP chemists may also be involved in regulatory affairs, working with government agencies to ensure that their products comply with all relevant laws and regulations.
The role of QP chemist generally requires at least a bachelor’s degree in chemistry or a related field. Some companies may also require experience in a particular type of QC lab work or knowledge of specific software programs used for QC purposes.
How Do You Calculate the Reaction Quotient?
In order to calculate the reaction quotient (Q), you must first determine the concentrations of all reactants and products present in the reaction mixture. Once you have this information, you can plug it into the following equation:
Q = ([products]/[reactants])
For example, let’s say we have a reaction where A and B are reactants and C and D are products. The concentration of each species is as follows: [A] = 0.5 M, [B] = 1.0 M, [C] = 2.0 M, and [D] = 1.0 M. Plugging these values into the equation above gives us a Q value of 4.0 for this reaction mixture.
It’s important to note that the Q value can change as a reaction progresses since concentrations are constantly changing as reactants are consumed and products are formed.
This is why Q is often used to predict the direction in which a reaction will shift in order to reach equilibrium – if Q > K (the equilibrium constant), then the products will be favored over reactants and vice versa if Q < K .
How Do You Find the Quotient of a Qp Reaction?
In order to find the quotient of a QP reaction, you will need to know the following information:
-The concentration of each reactant and product
-The rate of the forward and reverse reactions
-The equilibrium constant for the reaction
With this information, you can then use the following equation to calculate the quotient:
How Do You Find Partial Pressure from Qp?
When it comes to finding the partial pressure of a given gas, you will first need to determine the molar density of the gas. To do this, simply divide the number of moles of the gas by the volume that it occupies. Once you have determined the molar density, you can then use this value to find the partial pressure of the gas.
To do this, simply multiply the molar density by the universal gas constant (R). This will give you a value for P/V, which is effectively the partial pressure of the given gas. From here, all you need to do is multiply this value by V (the volume that the gas occupies), and you will have your answer for QP (the partial pressure of your chosen gas).
Chemical Equilibrium Constant K – Ice Tables – Kp and Kc
What is Qp in Chemistry
In order to understand what Qp is in Chemistry, it is first important to know what p stands for. In Chemistry, p stands for the partial pressure of a given gas. The partial pressure is the pressure that would be exerted by a particular gas if it were present alone in a container.
Qp then stands for the amount of heat required to change the temperature of a system when the partial pressure of a gas is changed.
This value can be used in order to find out how much heat is needed in order to change the temperature of a system when different gases are added or removed. This can be useful information for things like finding out how much energy is needed to produce certain chemicals.
It can also be used in order to figure out the best way to store chemicals, as well as how they will react with one another.
How to Calculate Q for Equilibrium
In order to calculate Q for equilibrium, you need to know the reaction’s K value and the concentrations of all reactants and products. Once you have this information, you can use the equation:
Q = Kc(products) / c(reactants)
To find Q, start by plugging in the known values for K and the concentrations of products and reactants. Then, solve for Q. For example, let’s say that we have a reaction with a K value of 1.2 and the following concentrations:
0.5 M for A
0.5 M for B
1 M for C
1 M for D
How to Calculate Q Heat in Chemistry
In order to calculate the Q heat in chemistry, you will need to know the values for the following variables:
– The starting temperature (T1)
– The ending temperature (T2)
– The amount of time (t) that elapses during the heating process
– The specific heat capacity of the material being heated (Cp)
With this information, you can use the equation Q = Cp x (T2 – T1) x t to calculate the Q heat.
For example, let’s say you wanted to calculate the Q heat for a piece of metal that is heated from 20 degrees Celsius to 80 degrees Celsius over a period of 10 minutes. The specific heat capacity of metal is 0.5 J/g°C. Therefore, using our equation we would have:
Q = 0.5 x (80-20) x 10
Q = 0.5 x 60 x 10
How to Find Q in Chemistry Thermodynamics
In thermodynamics, Q is a measure of the heat transfer that takes place during a chemical reaction. It is defined as the change in enthalpy (H) divided by the change in temperature (T). The units of Q are joules per mole (J/mol), and it is a fundamental parameter in many calculations involving chemical reactions.
To calculate Q, you need to know the values of H and T for the reaction you are interested in. These values can be found in tables of thermodynamic data or can be measured experimentally. Once you have these values, you can use the following equation:
Q = ΔH / ΔT
Where ΔH is the change in enthalpy and ΔT is the change in temperature.
It is important to note that Q is a value that depends on both the reactants and products of a reaction, as well as the conditions under which the reaction takes place.
This means that it is not possible to determine Q for a given reaction without knowing all of these other factors. However, once all of these factors are known, calculating Q becomes relatively straightforward.
Conclusion
If you want to know how to calculate Qp in chemistry, then you’ve come to the right place. In this article, we’ll show you how to do just that. First, let’s take a look at what Qp is and why it’s important.
Qp is short for “q-prime.” It’s a number that chemists use to describe how reactive a molecule is. The higher the Qp, the more reactive the molecule.
That’s because molecules with a high Qp are more likely to undergo chemical reactions.
Now that we know what Qp is, let’s learn how to calculate it. To do that, we need to know two things: the energy of the molecule and the rate of the reaction.
The energy of the molecule is measured in electron volts (eV). The rate of the reaction is measured in moles per second (mol/s). To calculate Qp, we simply divide these two numbers:
Qp = eV / mol/s
So there you have it! Now you know how to calculate Qp in chemistry.